I’m a PhD student in Materials Science & Engineering at UCLA studying battery materials under Professor Bruce Dunn working on a variety of projects:
1) Understanding the mechanism of lithium plating underneath coatings as an approach to circumventing lithium dendrites
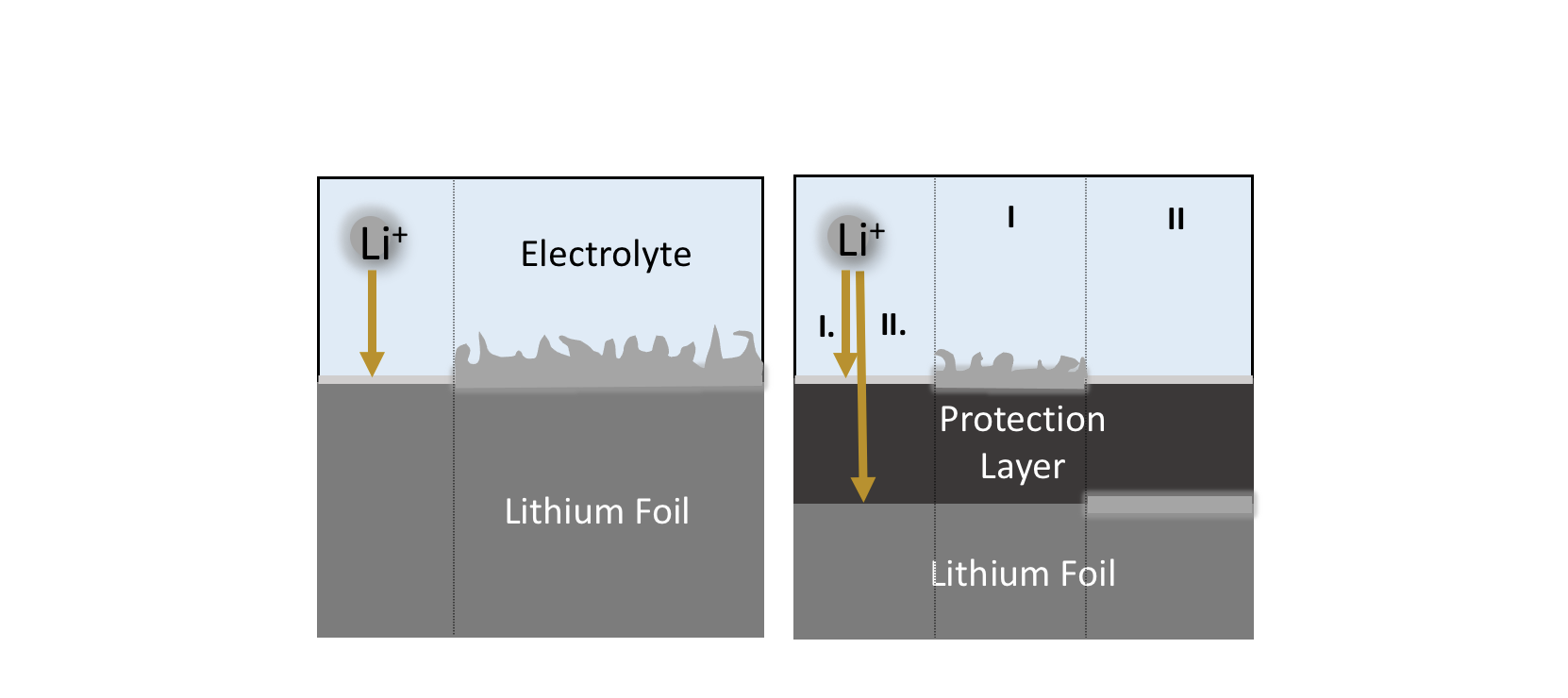
Lithium metal has long been considered the anode of choice for next-generation high energy density batteries due to its low standard reduction potential (-3.04 V vs. SHE) and high specific capacity (3860 mAh g-1, ten times that of commercially used graphite). The challenge of using a lithium metal anode stems from safety concerns in addition to long term stability/performance (Low Coulombic Efficiency and Dead Lithium). Both concerns can more or less be pin pointed at the formation of “lithium dendrites,” that is, finger-like and branch-like lithium structures that form and grow upon repeated plating and stripping cycles. Lithium dendrites have been found to puncture through separators and reach the cathode, resulting in a short circuit (large currents, high heat, flammable electrolyte= big safety issue). In order to address the problem, the interface, a critical region where breakdown and decomposition often occurs, must be properly understood and stabilized.
The use of coating layers on the lithium surface have been found to show promise in suppressing dendrite formation to some degree by tuning the morphology of the plated lithium. However, while most lithium coatings result in plating on top of the coating whereby the coating serves as a nucleation substrate, less studied is the case where the lithium can be transported through the layer and deposited underneath. The basic idea is that by confining lithium deposition to take place underneath the coating, we can effectively suppress lithium dendrite formation at the surface. The ability to understand the mechanism of plating underneath is both of fundamental and practical interest in better understanding the critical parameters to drive lithium transport through a layer and the ability to apply design principles to create a layer that can supress lithium dendrite growth.
For more details, you click here for an extended overview of the project here.
Here’s a link to a Perspective my collaborator Qizhang and I helped write in Applied Physics Letters about interface engineering of the challenging lithium metal interface and our two cents on where we see the field going.
Here’s a link to my work studying the mechanism of plating lithium underneath lithium-tin based intermetallic coatings as an approach to circumvent lithium dendrites published in Journal of Materials Research
I presented at the Materials Research Society (MRS) Fall 2020 Conference at symposium EN03 (Overcoming the Challenges with Metal Anodes for High-Energy Batteries) and my talk was presented with a “Best Presentation Award.”
2) Studying the influence microstructure on electrochemical performance of ionogel electrolytes.
Ionogels are a class of pseudosolids. They are a composite of two phases: 1) a room temperature molten salt(think liquid soup consisting of just ions) which is confined in 2) a solid silica matrix (think porous glass with pore sizes in the nanoscale). Ionogels behave like solids at the macroscale and like liquids at the nanoscale, resulting in faster ion movement(ions in liquids are generally faster than in solids), while imparting the beneficial mechanical properties of a solid. Solid state electrolytes often suffer from high resistances formed at the solid/solid interface. With an ionogel, we can circumvent this problem of high interfacial resistance due to the formation of solid/liquid interfaces from the ionic liquid. The future of battery technology has shifted towards an all solid state system, removing the need to use flammable liquid electrolytes. Ionogels are generally non-flammable due to their negligible vapor pressure which make them promising candidates from a safety standpoint. In addition to the fact that the ionic liquid does not evaporate out of the silica matrix, it also stays confined inside the host silica matrix through capillary forces which make them an interesting class of materials to study for more reasons than one. Lastly, there is a growing interest in the use of ionogels in low temperature applications(space perhaps?) due to the low freezing point which can further be lowered by mixing of different salts(entropy effect) and also due the confinement effects from the nanoporpous silica matrix.
I don’t have any published work on this project but if you’re interested in ionogels, I’d highly recommend this paper published in Joule from our group.
3) Translating photolithographic cleanroom technologies to fabricate a solid state photopatternable battery for on-chip applications
Photolithography, a process which uses UV-light (wavelength typically around 200-300 nm) to create nanoscale patterns and features, have revolutionized modern day electronics. Further, the growing field of the internet-of-things (IoT), highlights the need to better integrate batteries into the small device footprints while maintaing their high energy densities. Therefore, the ability to develop on-chip batteries is an attractive direction to achieve this. Silicon, which is the bread and butter of most traditional semiconductor technology also happens be a material that accomodate lithium through electochemical alloying (LixSi, Li15Si4, etc). In addition to it’s high capacity, the ability to pattern and create 3 dimensional Silicon architectures opens the door for a wide variety of on-chip battery and high power applications due to the increase surface area that 3D architectures offer over traditional planar designs.
For on chip-batteries, finding a suitable solid electrolyte, is an area of research that offers many possibilities. Previous research in the Dunn group has developed a photopatternable Li-ion solid electrolyte through modifying a commercial photoresist (SU-8) through the addition Li ion carriers via lithium salt. The easy processing and photopatternability in addition to the excellent thermal, mechanical, and electrochemical stability of the SU-8 based solid electrolyte presents a direction towards achieving on-chip batteries.
If you’re interested in this work, I’d check out this paper published in Advanced Materials from our group.

While the photopatternable solid electrolyte provides a good direction towards on-chip battery realization, the relatively low ionic conducitivty (52 uS/cm), prevents its use in high power applications. While adding more salt (hence Li-ions) into the SU-8 could incrementally improve the conductivity, there is a tradeoff with photopatterability as LiClO4 salt scatters the UV light during exposure of the photoresist. An alternative direction is the ability to create a photopatternable separator which can confine much higher ionic conductivity liquid electrolytes. The ability to spatially pattern and fabricate porous conformal separators is a direction that provides a new direction for enabling high power density on-chip batteries.

If you’re interested in this work, I’d check out this paper published in Advanced Materials from our group.
Lastly, if you are interested in reading the works linked above but have trouble accessing it, shoot me an email!
More Information
For a list of publications, click here.
This site was created using Jekyll Now which is a nifty, free tool that helps regular people like me make websites like this :)